When copper is dipped in the solution of silver nitrate, the solution turns blue. Give the reason along with chemical equation?
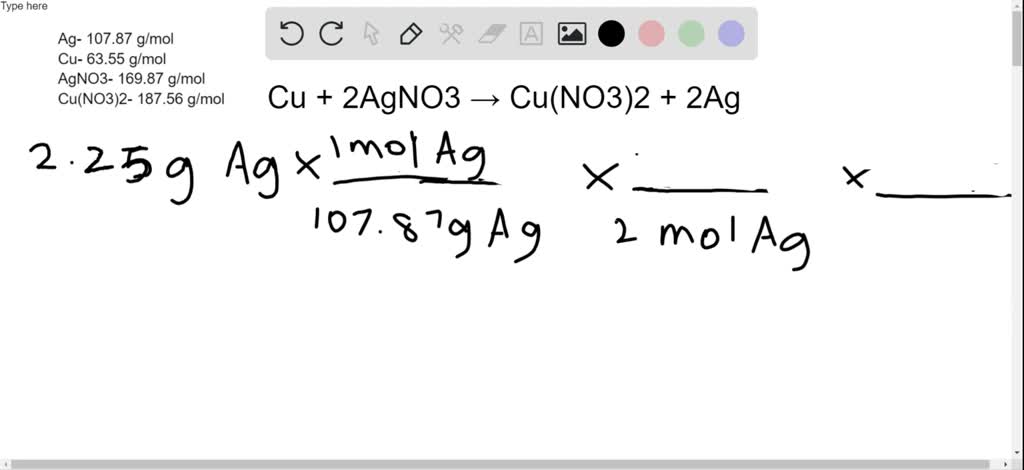
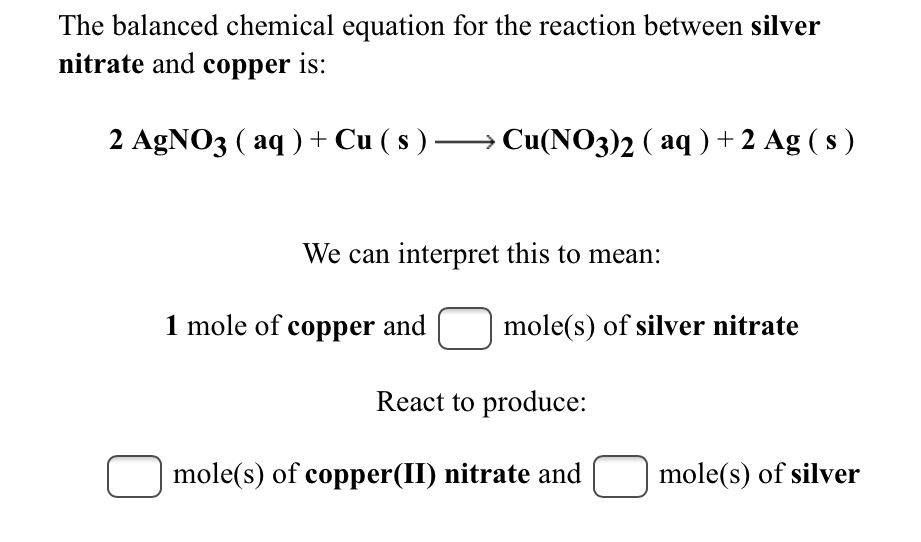
SOLVED:Copper reacts with silver nitrate through single replacement. a. If 2.25 g of silver are produced from the reaction, how many moles of copper(II) nitrate are also produced? b. How many moles
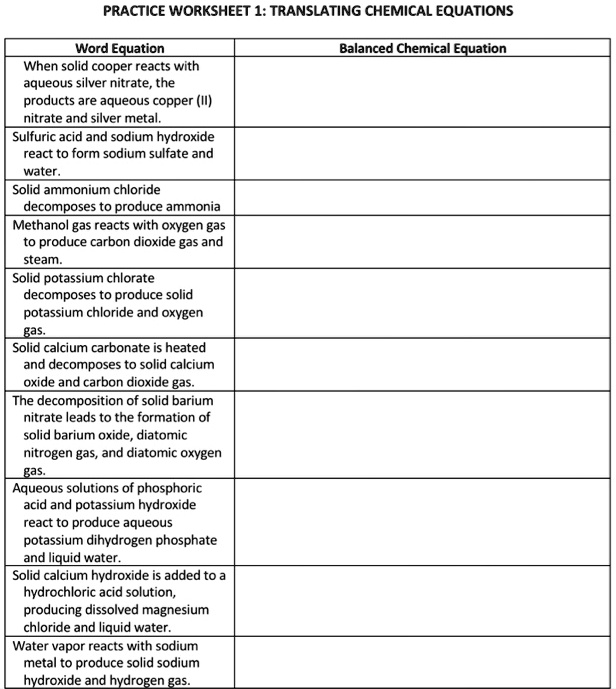
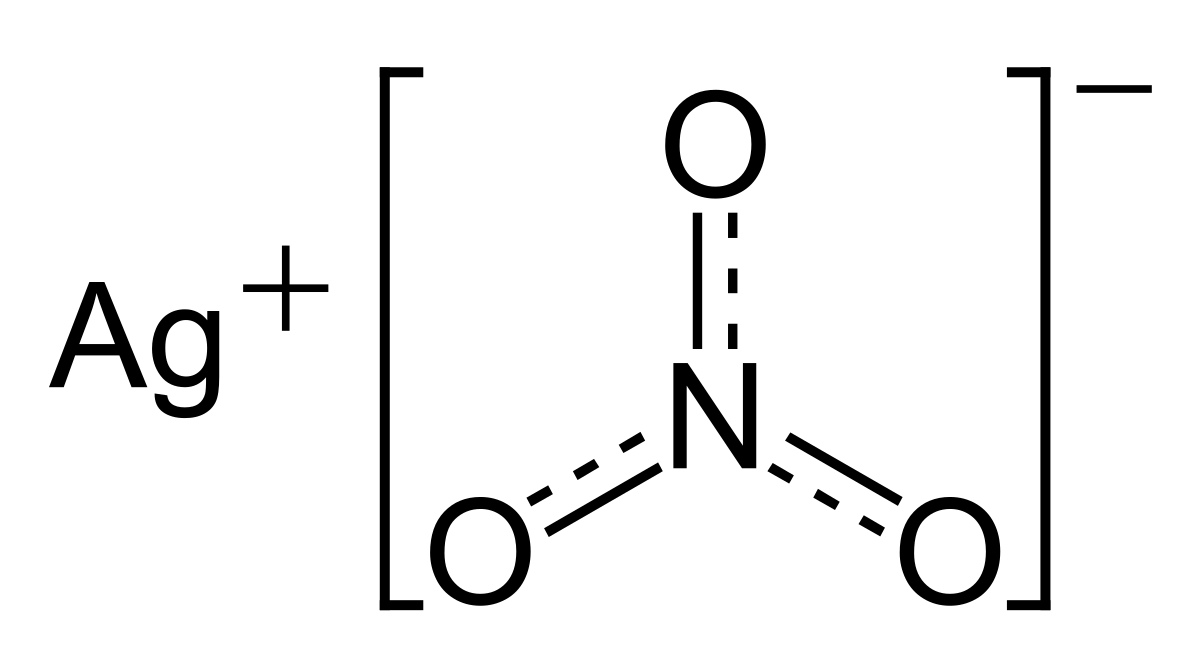
SOLVED: PRACTICE WORKSHEET 1: TRANSLATING CHEMICAL EQUATIONS Word Equation Balanced Chemical Equation When solid cooper reacts with aqueous silver nitrate the products are aqueous copper (II) nitrate and silver metal. Sulfuric acid

Word Equations & Predicting Products Mrs. Cook. Write a balanced chemical equations for this reaction. A solution of barium hydroxide reacts with a sulfuric. - ppt download

Write the balanced chemical equations for the following reaction:Calcium hydroxide + Carbon dioxide → Calcium Carbonate + Water.












/copper-wire-immersed-in-silver-nitrate-causing-blue-colour-81991997-582f14595f9b58d5b1a9b484.jpg)