Guidance for Industry Acute Myeloid Leukemia: Developing Drugs and Biological Products for Treatment

Designing Dose-Finding Phase I Clinical Trials: Top 10 Questions That Should Be Discussed With Your Statistician | JCO Precision Oncology

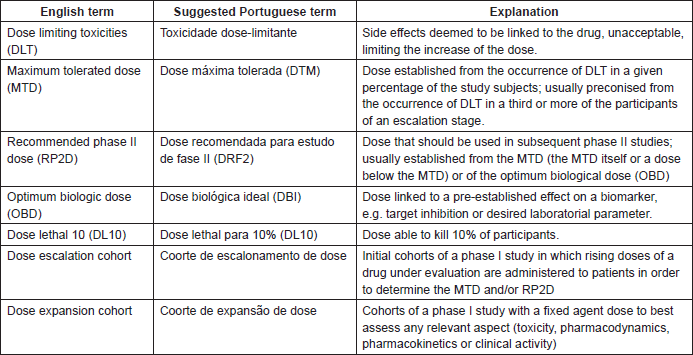
Towards new methods for the determination of dose limiting toxicities and the assessment of the recommended dose for further studies of molecularly targeted agents – Dose-Limiting Toxicity and Toxicity Assessment Recommendation Group

On Biostatistics and Clinical Trials: Dose Limiting Toxicity (DLT) and Common Toxicity Criteria (CTC) / Common Terminology Criteria for Adverse Events (CTCAE)

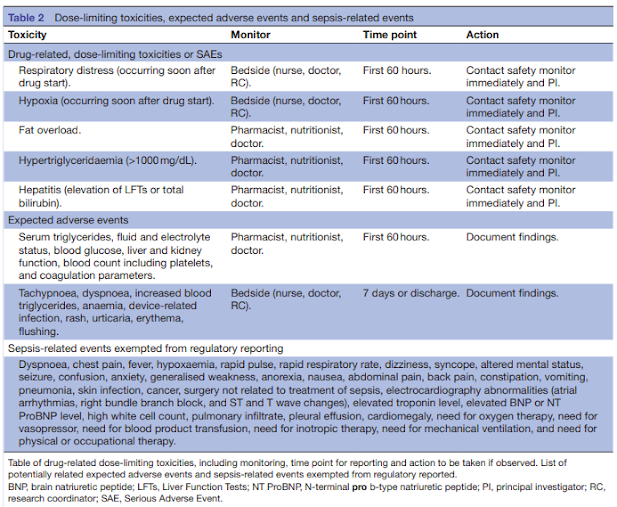
Design and Conduct Considerations for First‐in‐Human Trials - Shen - 2019 - Clinical and Translational Science - Wiley Online Library

Illustration of the chronic dose-limiting toxicity (DLT) concept. (*)... | Download Scientific Diagram

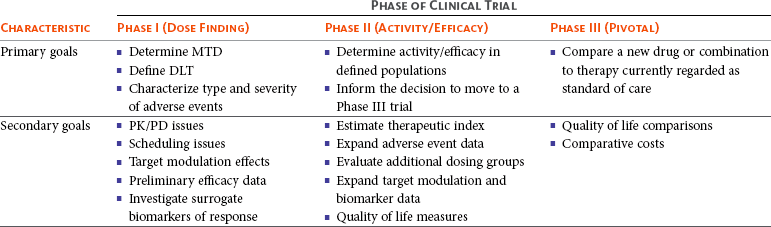
Moving Beyond 3+3: The Future of Clinical Trial Design | American Society of Clinical Oncology Educational Book

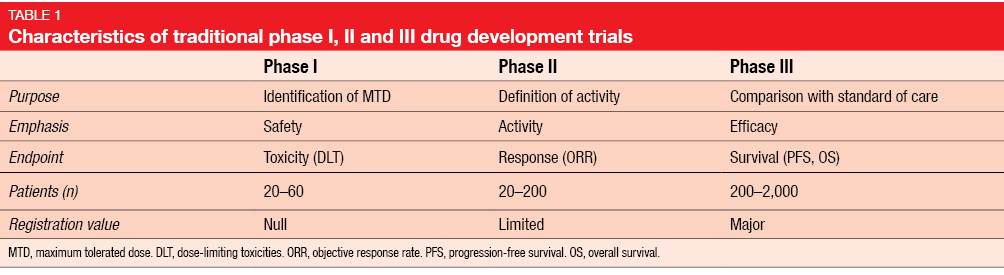
Prediction of Drug Approval After Phase I Clinical Trials in Oncology: RESOLVED2 | JCO Clinical Cancer Informatics

Designs of drug-combination phase I trials in oncology: a systematic review of the literature - Annals of Oncology