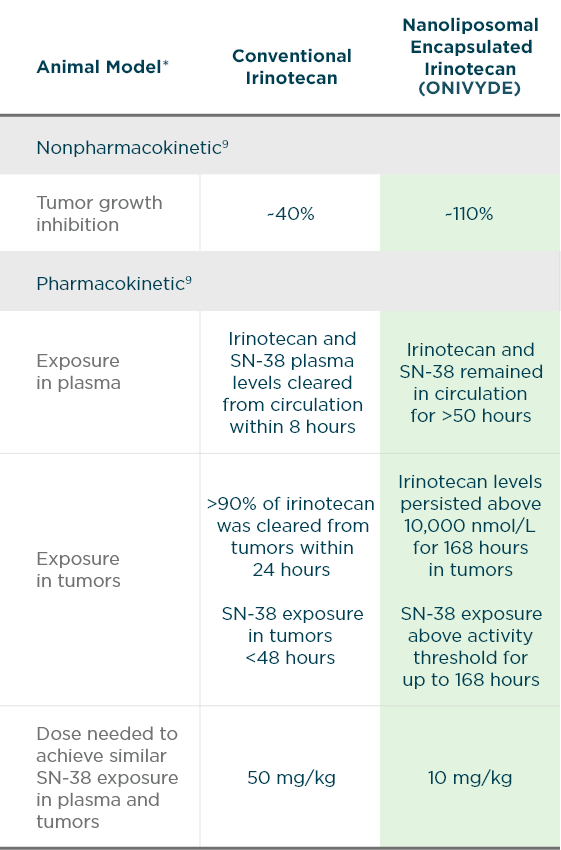
Pharmacokinetics | ONIVYDE® (irinotecan liposome injection) | Metastatic Pancreatic Cancer | HCP | Onivyde

DEP® irinotecan synergistic with Lynparza® in refractory human colon cancer model | BioMelbourne Network

Pre-therapeutic UGT1A1 genotyping to reduce the risk of irinotecan-induced severe toxicity: Ready for prime time - European Journal of Cancer

JCM | Free Full-Text | Safety, Feasibility and Technical Considerations from a Prospective, Observational Study—CIREL: Irinotecan-TACE for CRLM in 152 Patients

All You Need to Know About UGT1A1 Genetic Testing for Patients Treated With Irinotecan: A Practitioner-Friendly Guide | JCO Oncology Practice
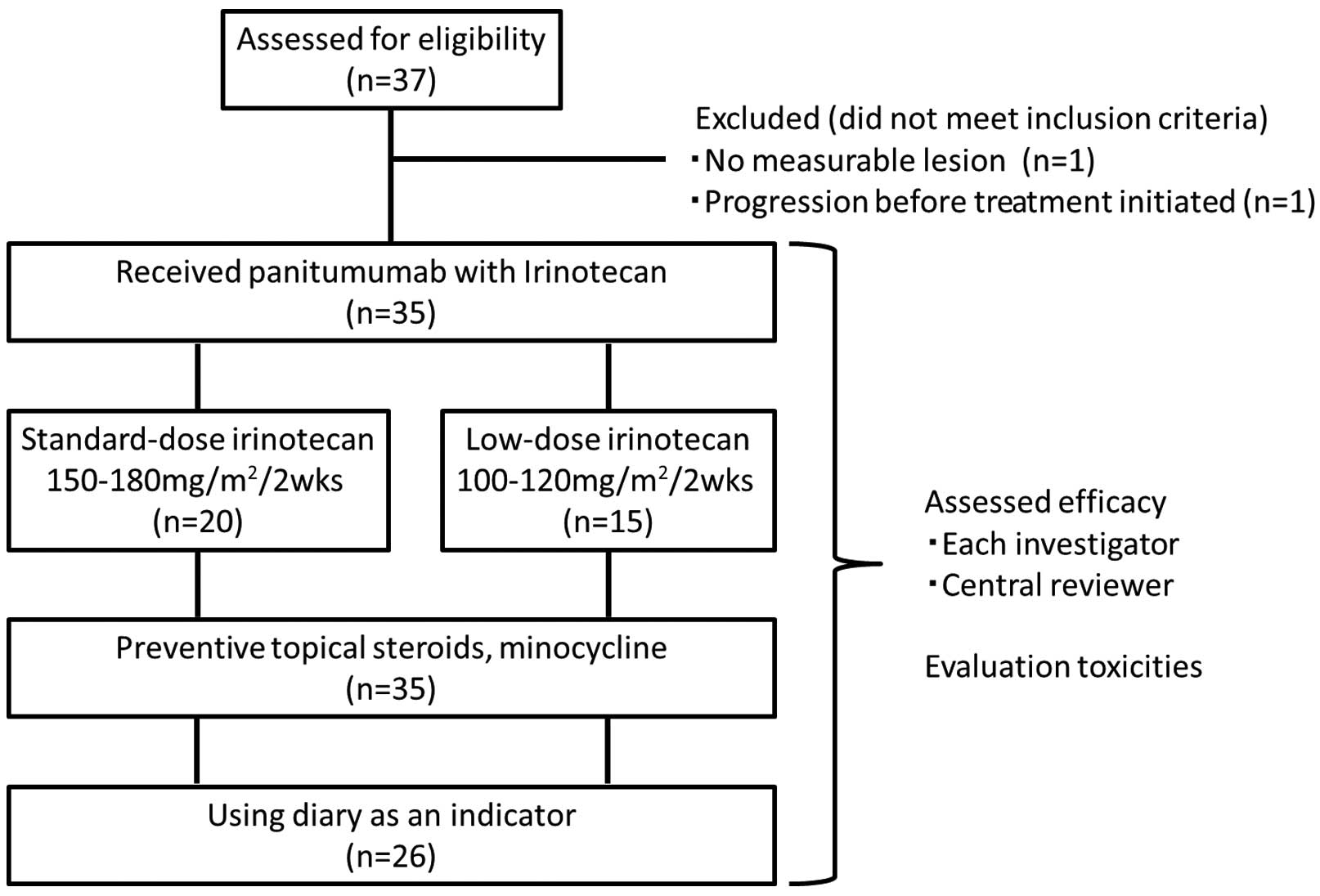
Phase II trial of panitumumab with irinotecan as salvage therapy for patients with advanced or recurrent colorectal cancer (TOPIC study)

Phase I study of cisplatin, irinotecan, and epirubicin administered every 3 weeks in patients with advanced solid tumours | British Journal of Cancer

UGT1A1*28 genotype and irinotecan dosage in patients with metastatic colorectal cancer: a Dutch Colorectal Cancer Group study | British Journal of Cancer
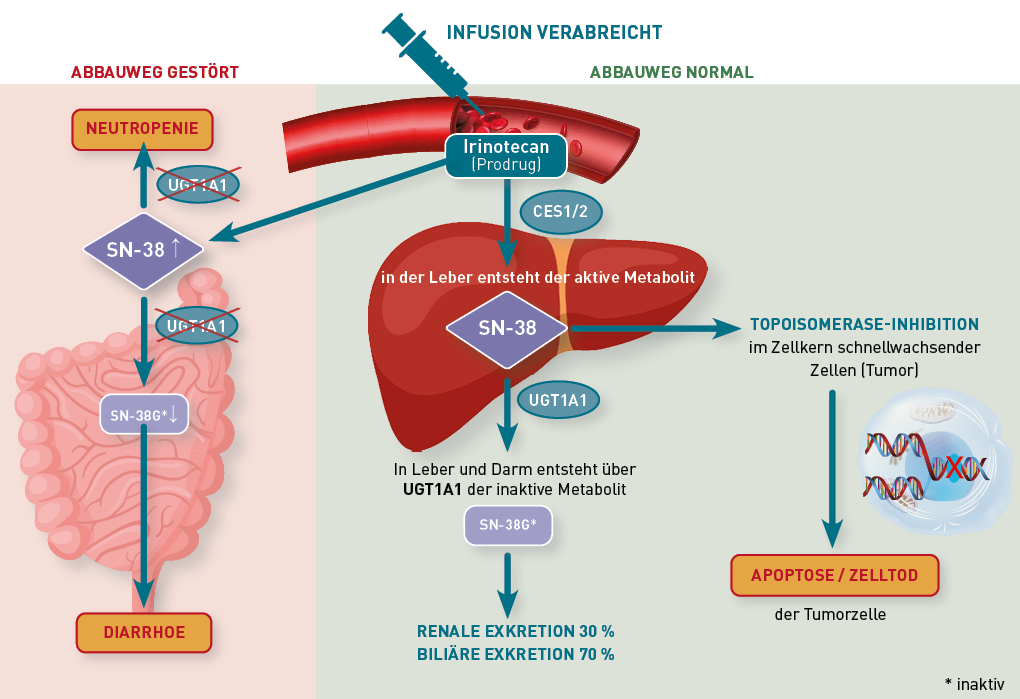
359 Erhöhte Irinotecan-Toxizität bei reduzierter UGT1A1-Aktivität - IMD Institut für medizinische Diagnostik, Labor